how to prepare a solution for a polarimeter|polarimetry pdf : inc 1.0 Purpose: The purpose of this SOP is to describe the procedure for operation, cleaning, and calibration of the polarimeter. 2.0 Scope: This SOP is applicable to the polarimeter available in the Quality control laboratory at the . 26 de set. de 2023 · Seleção Brasileira. Eliminatórias Copa do mundo. Nacionais. Internacionais.
{plog:ftitle_list}
Resultado da Novinha gostosa. 672.9k 100% 11min - 360p. Comendo a gostosa. 4.1k 79% 36sec - 720p. Levando rola delicia. 7.5k 86% 1min 0sec - 720p. Morena .
describe the features and operation of a simple polarimeter. calculate the specific rotation of a compound, given the relevant experimental data.
Usually, the sample is dissolved in a solvent and the resulting solution is placed in .Preparing and running your sample: Most people prepare a sample of concentration 1 (10 mg/mL) or 0.5 (5 mg/mL). These numbers can be flexible, though. ⇒ Just make sure you’re .
1.0 Purpose: The purpose of this SOP is to describe the procedure for operation, cleaning, and calibration of the polarimeter. 2.0 Scope: This SOP is applicable to the polarimeter available in the Quality control laboratory at the . In a polarimeter (figure 2), plane-polarized light is introduced to a tube (typically 10 .
Follow these steps to prepare the sample: Dissolve the sample in a solvent that has a similar refractive index as the sample. Filter the solution to remove any impurities that could affect the reading. Allow the solution to reach room .Usually, the sample is dissolved in a solvent and the resulting solution is placed in the tube. There are important factors affecting the outcome of the experiment. Optical rotation depends .Clean the polarimeter tube, beaker, flask and measuring cylinder with water. Fill the polarimeter tube with distilled water. As you rotate the analyzer through 3600, you observe .Prepare solutions of increasing concentrations for one of the enantiomers and pour into the polarimeter tube. Be sure to rinse the polarimeter tube with distilled water between solutions. Test each of these solutions in the .
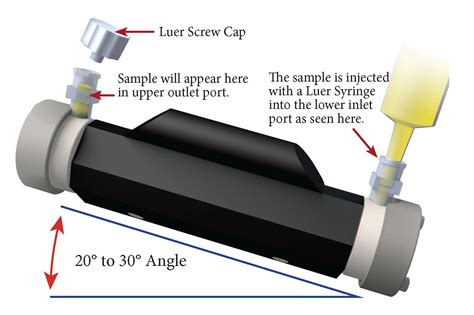
First, prepare a solution of your unknown substance with an appropriate solvent and note its concentration. To prepare the solution using your unknown substance, weigh out the desired mass of substance and using a funnel, .
The concentration and physical properties of the solution influence the plane of polarized light and this is detected as the angle of optical rotation by a polarimeter. From this measurement, different parameters can be defined, .In a polarimeter (figure 2), plane-polarized light is introduced to a tube (typically 10 cm in length, figure 3) containing a solution with the substance to be measured. . A solution with a known concentration (~0.5-3 %) of the . Determine the zero point of the polarimeter and then make five readings of the observed rotation of the test solution at 25°. Take an equal number of readings in the same tube with the solvent in place of the test .
Prepare 200 mL of a 30% sucrose solution. Fill the polarimeter tube with the sucrose solution such that the path length increases by 0.5 dm in length for each observation. Record the angles of minimum light intensity. These will be the .
Here is a diagram of a modern polarimeter. Image source: wikipedia . If you will prepare a solution with 0.300 mol of the R isomer, and 0.700 mol of the S isomer, what will be your prediction of the [𝛼] of the solution? Reply. Keith says: July 27, 2020 at 7:11 am. Was having a problem with this question in a textbook:• To prepare the solutions, pour the water into a 100 mL beaker and add your stir bar. Place the beaker on your stirring hotplate, and stir without heating. . • Repeat the previous step with the other sucrose solutions using your labmates’ polarimeter cells, but doing the measurements in your own polarimeter. After all of the students .The concentration and physical properties of the solution influence the plane of polarized light and this is detected as the angle of optical rotation by a polarimeter. From this measurement, different parameters can be defined, such as specific .The right hand corresponds to the reflection of the spatial arrangement. It is not possible to make the two hands congruent by arbitrary rotation. Many chemical compounds show the same behavior. Due to the lack of symmetry, these chemical compounds have an optical activity that can be measured with the polarimeter.
A polarimeter is an optical instrument with which one can accurately measure the angle by which the polarization of light is rotated e.g. when it passes through an optically active medium (containing chiral molecules). Operation Principle of Polarimeters. The basic operation principle of a polarimeter comprises the following: Using a Polarimeter and a demonstration of the physical phenomenon underpinning the technique. Using the polarimeter 1:45Demonstrating polarimetry with a las. Solution. The observed rotation of the mixture is levorotary (negative, counter-clockwise), and the specific rotation of the pure S enantiomer is given as dextrorotary (positive, clockwise), meaning that the pure R enantiomer must be levorotary, and the mixture must contain more of the R enantiomer than of the S enantiomer.
Surface Tension Meter service
• Prepare a sugar solution of known strength by dissolving the known amount of sugar (say 10 gm) into 100 ml of water. Take the polarimeter tube and remove the pure water. Fill it with the prepared sugar solution and again place it in the polarimeter.Polarimeters can be used in kinetics experiments to follow the change in concentration of an optically active sample as a reaction proceeds. Sugars are common examples of optically active compounds. Sucrose is a disaccharide that can be broken down into its two substituent monosaccharides, glucose and fructose. This process occurs too slowly in water to be . The polarimeter. The optical rotation is measured with an instrument called a polarimeter. In this apparatus, polarized light of known plane is emitted on one side of the instrument. . When studying a dilute solution, it is commonplace to report the solvent used for dilution as well as the concentration, in g/100 mL (or %), at which the .Study Notes. A polarizer is a device through which only light waves oscillating in a single plane may pass. A polarimeter is an instrument used to determine the angle through which plane-polarized light has been rotated by a given sample. You will have the opportunity to use a polarimeter in the laboratory component of the course. An analyzer is the component of a .
Note that this method is aimed at the measurement of test solution using a polarimeter which has been calibrated using a quartz plate. However, it serves to illustrate the special care needed in preparing sugar solutions. Weighing method It is possible to improve the accuracy in preparing sugar solutions by weighing the sugar and water.Prepare the solution of appropriate strength by dissolving a known amount of active substance in a known volume of distilled water. 7. Fill the polarimeter tube with the solution of known concentration. Note the rotation produced in by adjusting the scale and observing uniform illumination through the eye-piece. Saccharimeter: This type of polarimeter is used to measure the concentration of sugar in a solution. It is commonly used in the food and beverage industry. Automatic Polarimeter: This type of polarimeter uses a digital display to measure the degree of polarization. It is commonly used in pharmaceuticals and chemistry.

To determine the specific rotation of cane sugar solution using Polarimeter. SPECIFIC ROTATION: Specific rotation is the rotation produced by a column in a solution of an optically active . Weigh 5 gm of sugar and carefully dissolve it is water to make up to 100cc of solution. The solution should be well mixed by pouring it from one jar to .• To prepare the solutions, pour the water into a 100 mL beaker and add your stir bar. Place the beaker on your stirring hotplate, and stir without heating. . • Repeat the previous step with the other sucrose solutions using your labmates’ polarimeter cells, but doing the measurements in your own polarimeter. After all of the students . Solution Preparation for Polarimeter Calibration: 10% w/v Fructose Solution: Weigh accurately (5gm, it should be as accurate as possible) of previously dried fructose and transfer it in a clean and dry 50 ml volumetric flask, dissolve it in 25 ml of purified water. Make up the volume (50 ml) with purified water.
5.1.31 Discard the test solution from the polarimeter tube. 5.1.32 Switch ‘Off’ the mains. 5.1.33 Clean the polarimeter tube with distilled water if solutions are prepared in polar solution or clean the polarimeter tube with methanol or ether if the solvent is non-polar. 5.1.34 Keep the tube in the tube box. 5.1.35 Enter the details in the .In measuring optical rotation, plane-polarized light travels down a long tube containing the sample. If it is a liquid, the sample may be placed in the tube as a pure liquid (it is sometimes called a neat sample). Usually, the sample is dissolved in a solvent and the resulting solution is placed in the tube.1. Prepare 10 mL samples of 10%, 20%, and 30% solutions of D-Glucose in water. 2. Fill the cell with 10ml of distilled deionized water and place it into the polarimeter. Tilt the cell so that any remaining bubbles are caught in the Bubble Catch, which is the fat part of the cell. 3. Open the file named Polarimeter Introduction. 4. Start . Similarly prepare solution of 40, 30, 20 and 10g/ 100 ml by diluting 20.0 ml, 15.0 ml, 10.0 ml, and 5.0 ml of solution (A) to 25.0 ml with water . Measure the angle of rotation following steps of SOP and record the observation Annexure I. Take average of five readings for each concentration. Acceptance criteria for Polarimeter Calibration:
A polarimeter measures the direction and extent of the polarisation plane rotation. Depending on the type of instrument, the analyser is rotated manually or automatically until the maximum intensity of the light falls on the detector. A polarimeter is the entirety of light source, polariser, polarimeter tube, analyser and detector.The Preparation of Solutions. To prepare a solution that contains a specified concentration of a substance, it is necessary to dissolve the desired number of moles of solute in enough solvent to give the desired final volume of solution. \( Molarity of solution = dfrac{moles\: of\: solute}{Volume of solution} \tag{12.1.1}\) In measuring optical rotation, plane-polarized light travels down a long tube containing the sample. If it is a liquid, the sample may be placed in the tube as a pure liquid (its is sometimes called a neat sample). Usually, the sample is dissolved in a solvent and the resulting solution is placed in the tube.
polarimetry study guide pdf
25 de fev. de 2016 · Um Dia Você Aprende - William Shakespeare O Menestrel - YouTube. © 2023 Google LLC. Clique aqui e assista uma vídeo Aula Gratuita: http://bit.ly/EBCoerenciaUm Dia Você Aprende.
how to prepare a solution for a polarimeter|polarimetry pdf